HPH MRD Personalized Cancer Detection with Ultra-High Sensitivity
HPH MRD is a truly personalized cancer detection test using whole genome sequencing to inform the test design and manufacturing process, delivering an ultra-high sensitivity test for every patient.
HPH MRD is performed in Germany and samples and data remain local.
HPH MRD is based on the technology of Haystack MRD® by Quest Diagnostics®.
Superb ctDNA Detection Accuracy
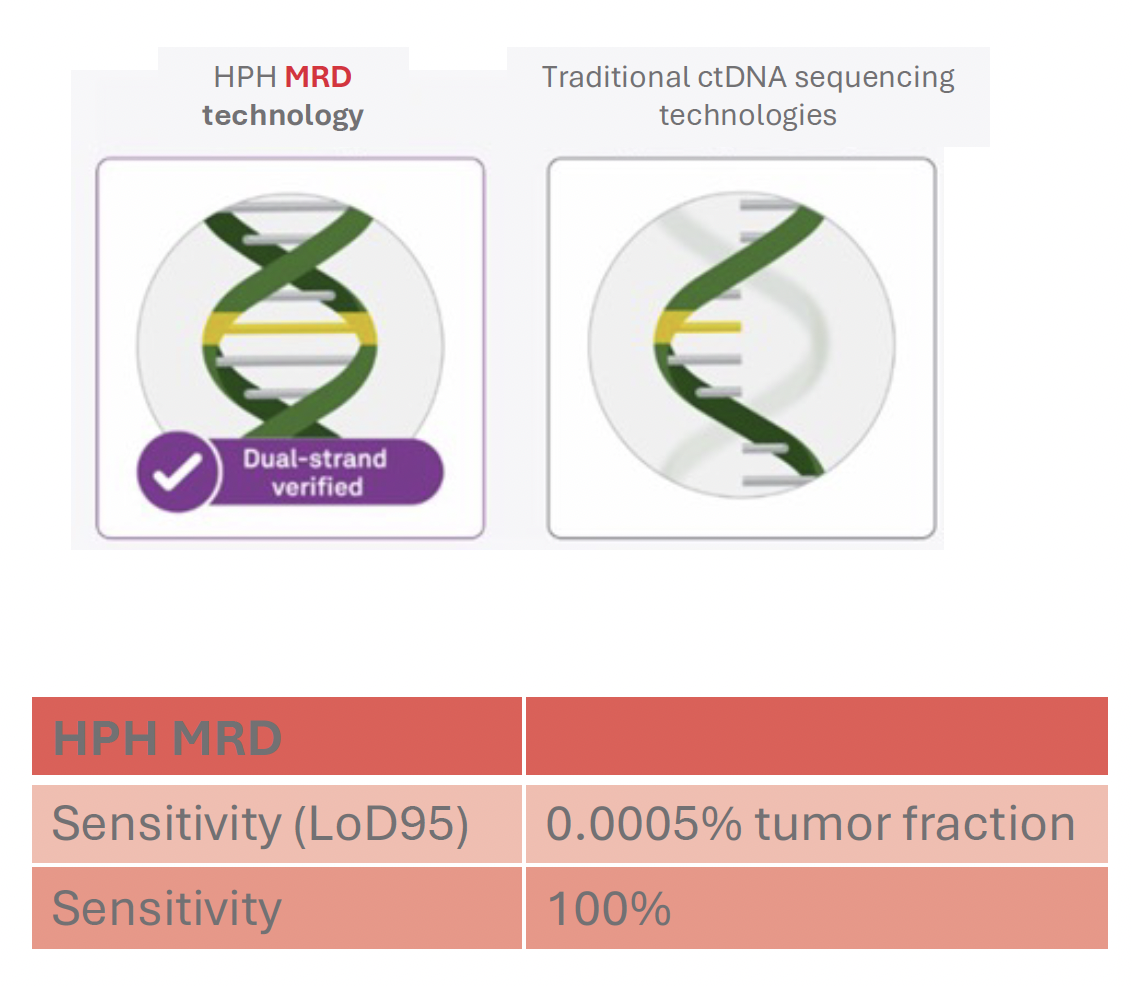
High accuracy detection of ultralow ctDNA levels is essential in MRD testing. Our HPH MRD test uses patented error-correction combined with advanced deep sequencing technology to double-check both DNA strands for each variant eliminating sequencing noise and delivering extraordinary sensitivity and specificity.
Whole genome sequencing to inform the test manufacturing process allows us to select the most reliable mutations to track ctDNA in blood samples of a patient. The selection and design process results in high sensitivity of our HPH MRD test.
Whole Genome Sequencing Technology
HPH MRD utilizes whole genome sequencing (WGS) technology to identify tumor-specific mutations.
The test is personalized for each patient based on their tumor's unique genetic profile, allowing for highly sensitive detection of ctDNA in blood samples.
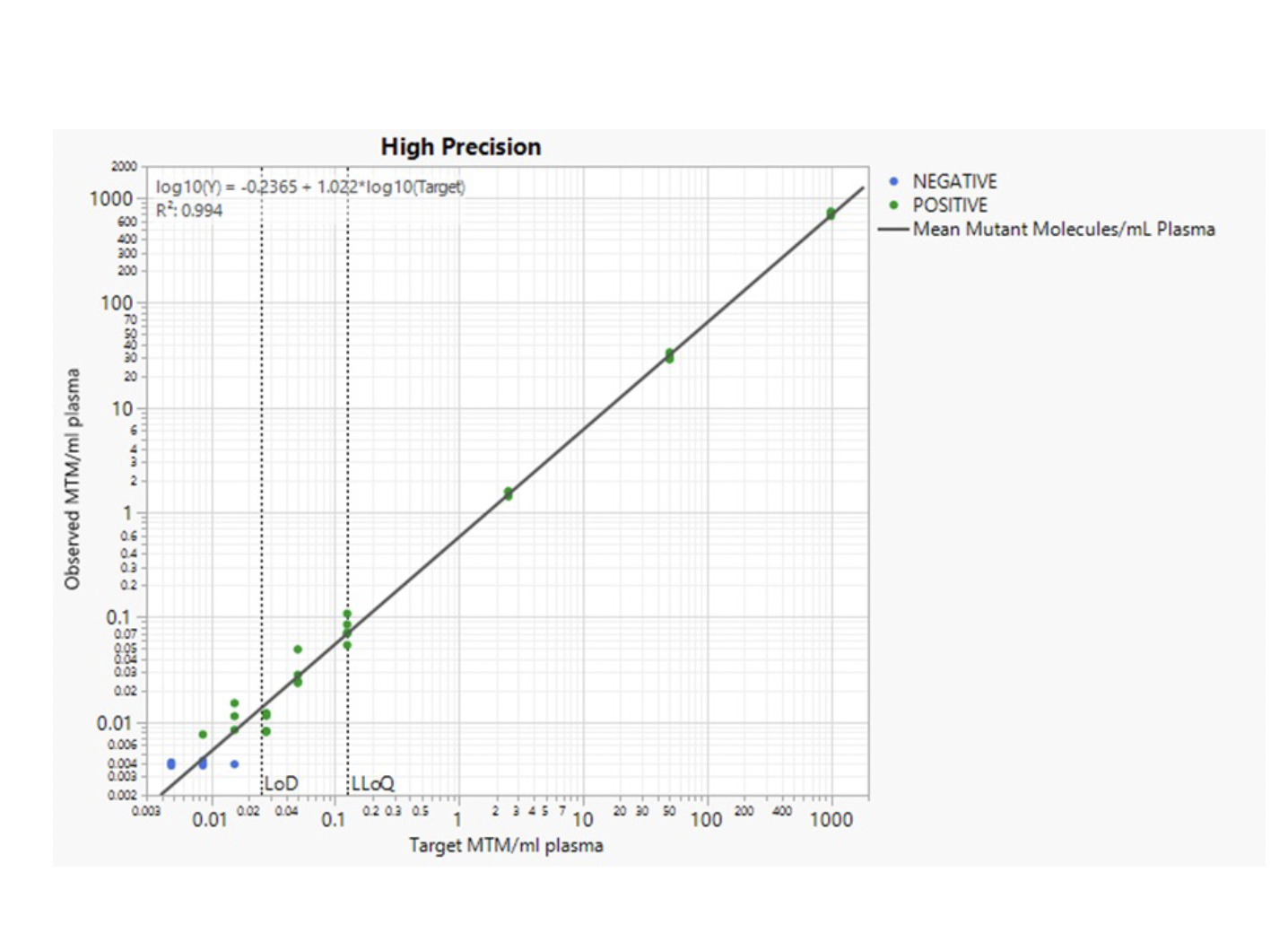
Ultra-High Sensitivity Detection Limits and Large Range of Quantification
HPH MRD can identify miniscule amounts of tumor DNA in a background of normal DNA and is thus ultra-sensitive. The limit of detection has been determined to be below 1 ppm (parts-per million), which means that HPH MRD can identify one tumor molecule in one million normal DNA molecules.
Additionally, HPH MRD has been verified to be quantitative in a range from 0.125 – 1000 mutant tumor molecules / mL plasma. This high level of quantitative precision allows you to compare results from different timepoints in the patient journey and monitor changes in ctDNA levels.
Tumor Specific Individual Test Design and Manufacturing
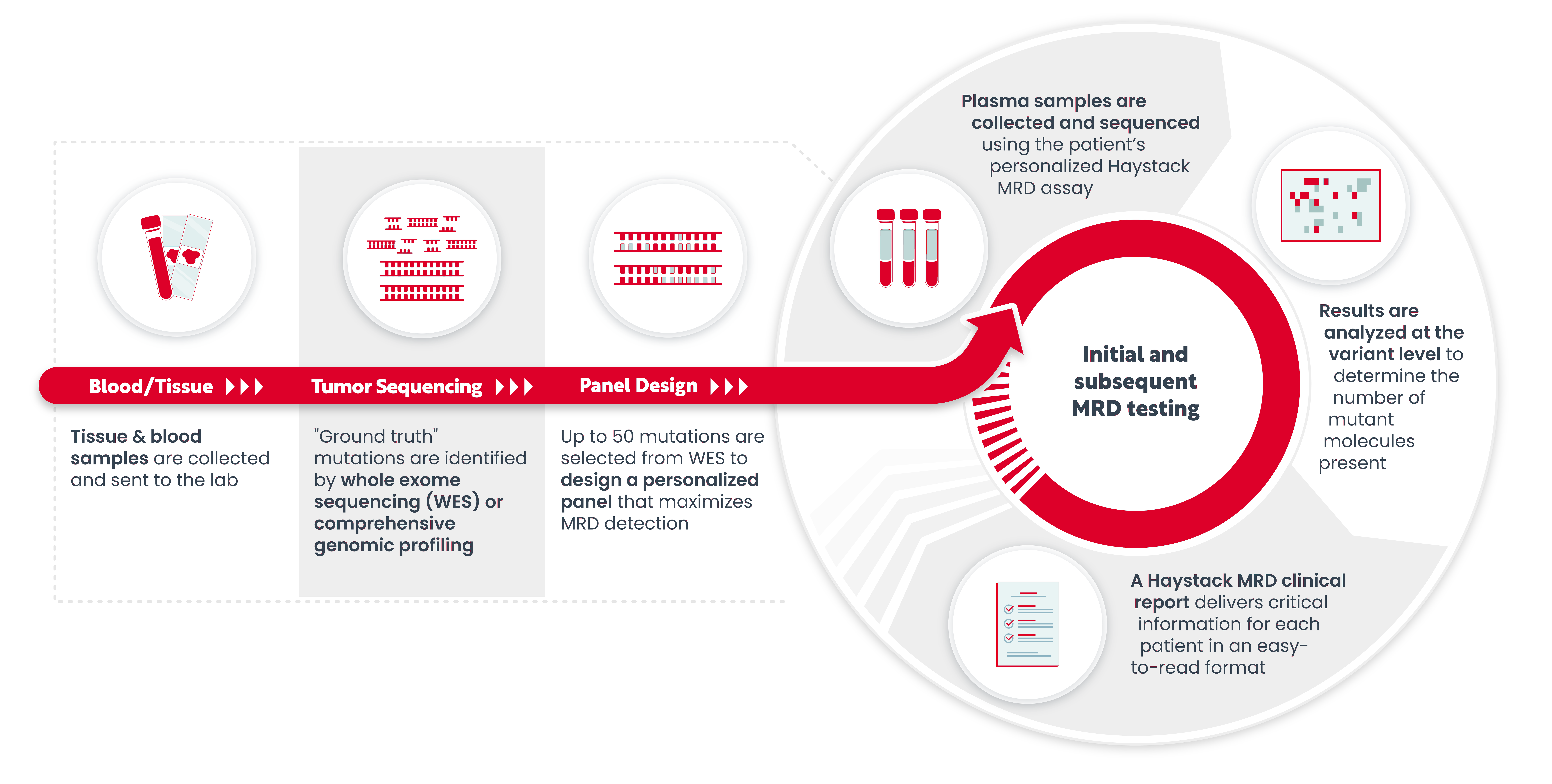
HPH MRD test design and manufacturing requires a tumor tissue and a blood sample for whole genome sequencing analysis. Once the samples have been sequenced and analyzed we are identifying up to 50 variants for an individual test manufacturing process.
The personalized test is then used to create the HPH MRD Baseline report on the initial test sample provided.
Follow up testing can be quickly ordered as the manufactured test is ready to use. Simply send in a new blood sample of the patient and we can work on the HPH MRD Monitoring report.
Clinical Use Case Indications

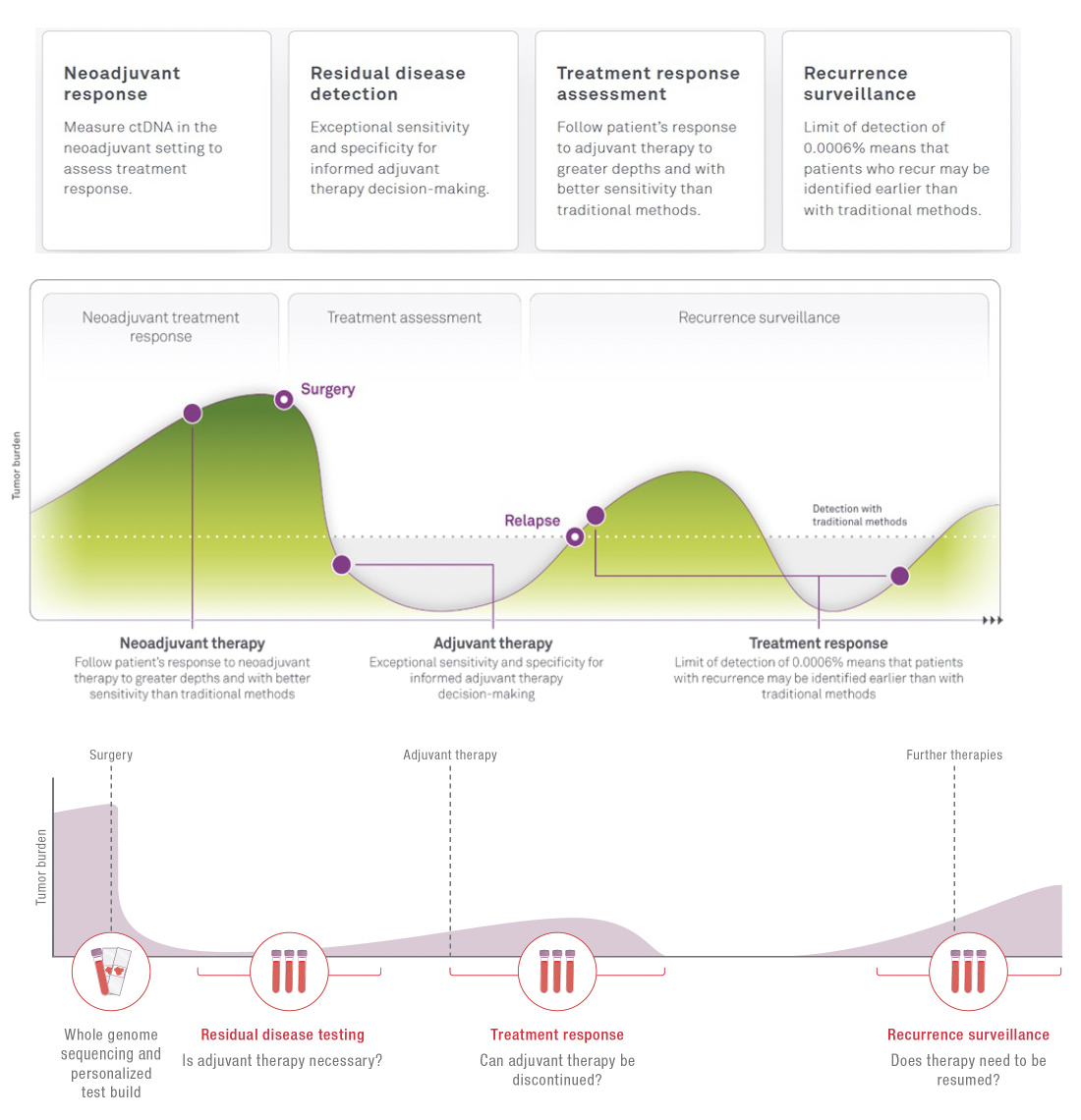
The high sensitivity and large quantification range of HPH MRD make it a useful tool in multiple settings along the patient journey. This ensures that whether ctDNA is detected or not, you can trust the results to help you more fully understand the disease status, delivering the confidence needed when answering most pressing questions.
Neoadjuvant treatment
Monitor the effectiveness of the treatment and inform the decision for the next steps.
Surveillance
Giving you confidence in your current situation. A simple blood draw at your local physician is sufficient to receive a new result.
Adjuvant treatment
Supporting the decision if adjuvant treatment is needed. Not every patient benefits from chemotherapy or radiotherapy. Treatment with an immune checkpoint inhibitor can be lengthy and you might want to know if cancer can still be detected.
Quality and Safety First
HPH is a DAkkS accredited laboratory and is thus compliant with the international ISO15189 standard for Medical Laboratories.
HPH MRD testing is taking place in Germany; data analysis, storage and samples remain local.
Clinical Studies Demonstrate the Usefulness of ctDNA as Biomarker in Cancer Testing
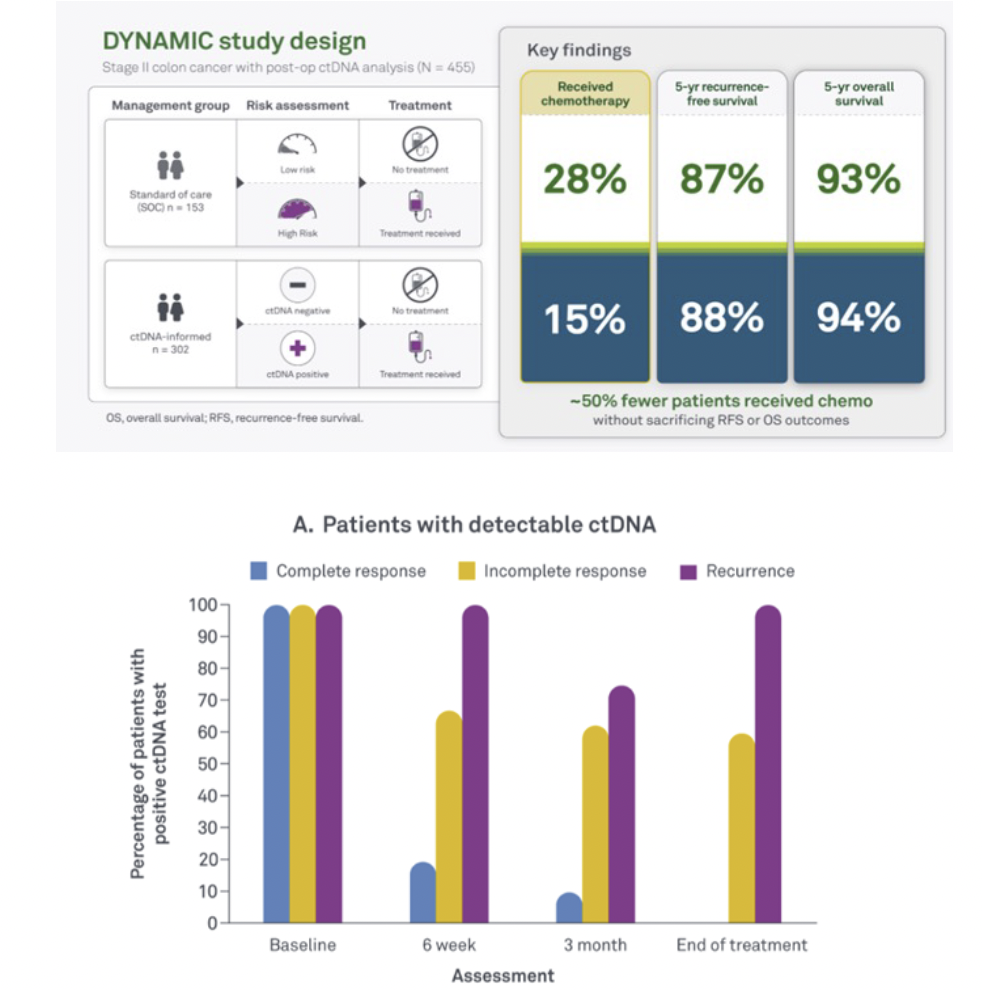
DYNAMIC study: The first interventional study to show that ctDNA can guide adjuvant therapy decisions. Results validated after 5 years of follow-up were generated using an earlier version of our technology.
Immunotherapy Treatment Response: In a landmark study, patients with mismatch repair–deficient (dMMR) cancers underwent immunotherapy alone (without surgery), chemotherapy, or radiation. Our technology served as a real-time method to track patient's response to treatment. It identified response to therapy earlier than traditional imaging methods.
HPH MRD is based on Haystack MRD® technology by Quest Diagnostics.